 |
| https://upload.wikimedia.org/wikipedia/commons/1/13/Phoenicopterus_minor_-Lake_Bogoria%2C_Kenya-8a.jpg |
Lake Natron, Tanzania, Africa
 |
| https://oncirculation.files.wordpress.com/2013/11/petrorrific2.png |
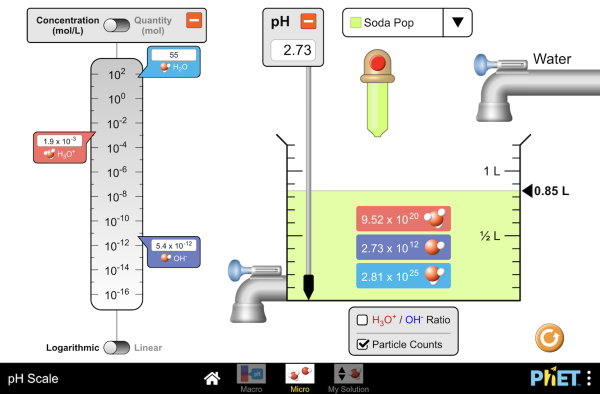
Making Sense Out of pH
Background Information:
Lake Natron, Tanzania has a pH of between 9 and 10 as a result of water from hot springs entering the lake. There is no outlet, water simply evaporates increasing the salt concentration of the lake. Acid rain does lower the pH of the lake, but in dry years the pH remains high. The red color of the water comes from microorganisms in the lake. Few other organisms are able to survive in these harsh conditions. Flamingos however, can withstand the high pH and use the lake as a breeding ground.
Student Directions: Use the Animation Below:
Group Performance:
- Investigate how adding water to the container of various liquids changes the pH of the liquids.
- Formulate questions and investigate explanations for the causes of the patterns you see.
- Develop evidence to support your explanations.
Individual Performance: (SSW)
- Write in your journal your explanation of this phenomenon. Include evidence to support your explanation for the cause of the change in pH.

No comments:
Post a Comment